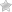Do-it-yourself chrome plating at home: technology, video

Chromium plated elements are typically used to enhance the design of cars and motorcycles. The cost of such parts manufactured in production conditions is quite high, but chrome plating at home is quite possible. By applying chrome to the surface of various products with your own hands, you can save significant financial resources.
Compliance with the technology will allow you to obtain good quality chrome surfaces at home
Many craftsmen who are not indifferent to the appearance of their vehicle are showing interest in doing chrome plating of various parts with their own hands at home. There are many nuances in chrome plating technology at home. In addition, it requires strict adherence to the sequence of all its stages.
Preparation for the procedure
To perform chrome plating at home, you need to carry out some preparation. First of all, you should choose the right premises (preferably non-residential). In addition, before performing chrome plating, you must:
- ensure ventilation of the room in which the technological operation will be performed (it is desirable that such ventilation is not natural, but forced);
- find personal safety equipment (respirator, safety glasses, thick rubber gloves and apron);
- resolve the issue of how process waste will be disposed of.
A homemade chrome plating bath can be made from thick vinyl plastic
Design of chrome plating plant
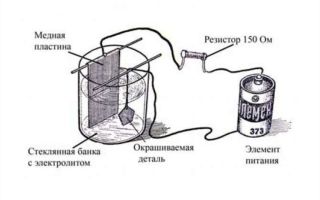
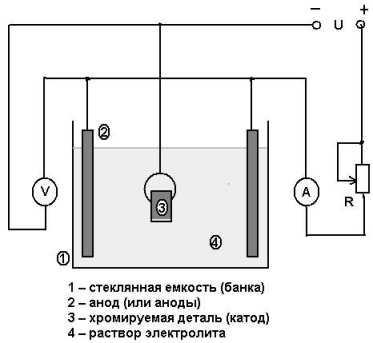
For chrome plating at home, you can make a device consisting of the following elements:
- container in which the container for chrome plating will be installed;
- the container itself, which can be used as a 3-liter glass jar;
- a wooden box with insulated walls that facilitates the high-quality electrolysis process (fiberglass, sand, mineral wool or glass wool can be used as insulating elements for the walls of such a box, which should have the effect of a thermos);
- a heating element, which can be a conventional heating element of appropriate power;
- a device with which you can measure temperature (it is best to use a contact thermometer, which allows you to automate the technological process);
- a sealing cover, which is best made from wood board or moisture-resistant plywood;
- wires, with the help of which the workpiece is connected to the negative contact of the electric current source, and the anode, also immersed in an electrolytic solution, to the positive one (the wire connected to the workpiece is best equipped with an alligator clip: in this case it will be connected much more convenient).
Installation diagram for chrome plating
Your chrome plating kit should also include a special bracket for hanging the workpiece in the electrolyte. It is necessary to use such a bracket so that the surface of the product is covered with a layer of chrome evenly on all sides.
Power Supply Requirements
Coating the surface of products with a layer of chrome, including chrome plating of parts at home, involves the use of a direct current source. The electrical circuit of a homemade chrome plating device must meet the following requirements.
- The current source that is equipped with the chrome plating kit must be able to adjust the output voltage. In its simplest form, a conventional rheostat can be used as such a control device.
- The cross-section of the wires with which the anode and cathode are connected to the current source is selected according to the maximum current strength. Thus, equipment used for chrome plating of small-sized products must be equipped with wires with a cross-section of at least 2.5 mm2.
- If a household device for chrome plating involves the use of a thermostat rather than a conventional thermometer, then for the correct use of such a measuring device it is necessary to assemble an appropriate electronic circuit.
Thermostat circuit
Composition of the electrolyte and rules for its preparation
If you decide to apply a layer of chromium to the surface of the product yourself, it is important not only to assemble the appropriate equipment, but also to properly prepare the electrolytic solution. The composition of such a solution for chrome plating of metal includes the following components:
- chromic anhydride (CrO3) – 250 g/l;
- sulfuric acid (H2SO4) – 2.5 g/l.
These components are mixed with water.
After mixing, use a hydrometer to check the density of the solution, which can be used to judge the content of chromic anhydride
There are certain rules for preparing an electrolytic solution, which are as follows.
- The container in which the electrolyte is prepared is half filled with water. If you use ordinary water rather than distilled water, it should be boiled and allowed to settle. The temperature of the water to which the remaining components are added should be about 60°.
- After the water has been prepared and heated to the required temperature, chromium anhydride is poured into it and the resulting mixture is stirred until it is completely dissolved.
- If the volume of the solution has decreased, it is necessary to add water.
- Sulfuric acid is poured into the resulting solution. It should be borne in mind that the above values for the H2SO4 content in solution are relevant for an acid with a specific gravity of 1.84.
- After the electrolyte is ready, it needs to be “worked out”. An electric current is passed through it for 3.5 hours, the strength of which is calculated using the following formula: 6.5 A for each liter of solution. Knowing the technical characteristics of the current source used, you can easily determine the required volume of electrolyte. If all steps to “process” the electrolytic solution are performed correctly, its color will change to dark brown.
- The electrolytic solution, with which you will chrome plating parts yourself, must be allowed to stand in a cool room for 24 hours.
How to prepare the workpiece
The quality and durability of the coating applied to it depend on how correctly and thoroughly the chrome surface is prepared. While the finished electrolytic solution is settling, you can start preparing the product, which consists of performing a certain list of actions.
Cleaning
During the cleaning process, not only traces of dirt, but also remnants of old paint, rust, and any other foreign layers are removed from the part processed before chrome plating.
The tools and devices used for cleaning are selected depending on how dirty the surface is.
To perform this procedure, in particular, both ordinary sandpaper and a grinding machine can be used.
Degreasing
The technology of chrome plating in production or at home requires mandatory degreasing of the surface being treated. The characteristics of the applied chrome coating also depend on the quality of this procedure.
For degreasing, as a rule, a special solution is prepared, which allows this procedure to be performed as efficiently as possible. The most popular of these aqueous solutions includes the following components:
- sodium hydroxide – 150 g/l;
- silicate glue – 5 g/l;
- soda ash – 50 g/l.
How is chrome plating done?
After all the preparatory work has been completed, a natural question arises: how to chrome a part correctly? First of all, it is necessary to heat the electrolytic solution to the required temperature (53±2°) and maintain it throughout the entire processing process. After this, the product is placed in an electrolyte, which should already contain the anode. After some time, during which the temperature of the product becomes equal to the temperature of the electrolytic solution, an electric voltage is applied to the anode and the product.
To uniformly cover the surface with chromium, it is necessary to correctly position the workpiece and the internal anode
After chrome plating is completed, the product is removed from the electrolyte and placed in a heating furnace, where it is subjected to heat treatment for 2.5 hours. This is necessary in order not only to improve the adhesion of the chromium layer to the surface of the base metal, but also to increase the hardness of the coating.
In order to perform decorative chrome plating with the highest possible quality, it is important to study theoretical material and learn the specifics of such a process (for example, from a video), as well as responsibly approach all stages of preparing the part and the electrolytic solution.
The duration of chrome plating and current density affect the thickness of the finished coating
Defects in chrome coating and the reasons for their occurrence
When doing chrome plating with your own hands, many home craftsmen are faced with the most typical defects in the applied coating, which can arise for various reasons.
1. There is shine on the coating, but it is uneven.
- The current supplied to the product and anode is too high.
- The temperature of the electrolytic solution has not been brought to the required value.
2. There is no shine at all on the applied coating.
- There is too much or not enough chromic anhydride in the electrolytic solution.
- The operating current rating has been exceeded.
- The amount of sulfuric acid in the solution is insufficient.
3. Brown spots appeared on the chrome surface.
- The amount of chromic anhydride in the electrolytic solution is exceeded.
- There is not enough sulfuric acid in the chrome plating solution.
4. Small pits are visible on the applied coating.
- The treated surface was not well prepared.
- When processed in solution, hydrogen is not removed from the surface. To solve this problem, you should change the layout of the product in the solution and the method of its subsequent drying.
5. Chrome plating is characterized by unevenness.
- The operating current has been exceeded.
6. The applied coating was too soft.
- The operating current is underestimated.
- The temperature of the electrolytic solution is higher than the required value.
7. The applied coating peels off.
- The voltage supplied to the anode and cathode is unstable.
- The surface of the workpiece was not sufficiently degreased.
- The electrolyte temperature decreased during processing.
Nuances of technology
The room for doing chrome plating yourself at home should not only be non-residential, but also quite spacious.
When carrying out preparatory activities, the following nuances must be taken into account.
- The polishing of the product performed before chrome plating should be of the highest quality.
- All chemicals used in the chrome plating process must be measured in precise quantities.
- To prepare the electrolyte, it is necessary to use only chemically pure sulfuric acid, but the issue of finding and purchasing chromic anhydride will have to be resolved separately, since it cannot be found on the open market.
Chromic anhydride is a reagent in the form of red-violet crystals. Volatile in air, hygroscopic, very strong oxidizing agent
For chrome plating, you need to select a direct current source whose power will be sufficient to process products of various sizes. Naturally, the container for the electrolyte must be of sufficient volume.
Source: http://met-all.org/obrabotka/prochie/hromirovanie-svoimi-rukami-domashnih-usloviyah-tehnologiya-video.html
Chrome plating at home: features and advantages of the technology

Chrome plating at home is not a very complicated technology that can be easily done with your own hands.
Having mastered the subtleties and nuances of working with various metals, you can save a lot on the services of specialists.
What features does this method have, and what variants exist for steel, copper and aluminum?
Methods for chrome plating parts
Chemical chrome plating is the layer-by-layer application of chromium to metal products. This is a great way to not only protect steel or aluminum from damage, but also improve the appearance and basic characteristics of the part.
There are several options for metallization methods, including:
- Galvanic.
- Chemical.
- Spraying.
It is important to consider that each method has certain nuances, and therefore it is necessary to select the optimal solution and appropriate equipment for copper, cast iron and steel.
Chrome plating of some materials requires a special approach. We are talking about aluminum - before making the main coating, it is worth taking care of the intermediate layer.
As additional protection, you can use special equipment to apply an anti-corrosion agent, which will extend the service life of the surface.
Galvanic method
Galvanic chrome plating is always popular.
The advantages of choosing this technology are obvious:
- Durability.
- Wear resistance.
- Increased hardness and strength.
- Resistant to any influences, including temperature changes and aggressive chemicals.
But the main value of galvanic coating is that even with prolonged heating it does not lose its shine and brightness of color. Sulfur compounds do not have a negative effect - it is possible to perform high-quality and durable decorative chrome plating.
The scope of application of galvanic coating is quite wide. This includes the creation of products with reflective properties, the application of a protective film, as well as the formation of a decorative coating, including the restoration of a worn surface to increase its service life.
To complete the task yourself, you will need to prepare in advance a special container that is highly resistant to acid.
As for the electrochemical method, it is based on the principle of electrolysis - current passes through a composition that includes acid, alkali, and chromium salts.
As a result, chromium cations are released, which coat the product. In this case, the temperature should vary from 50 to 60 °C, and the current density from 25 to 60 A/dm². The quality of the coating depends on the correct choice of basic parameters.
Chemical method
Chemical metallization is carried out on the basis of a special technology, which is carried out on the basis of a whole list of components. Among them:
- Water.
- Chromium chloride.
- Sodium hypophosphate.
- Sodium citrate.
- Acetic acid.
- Caustic sodium solution (20%).
It is important to consider that the procedure is performed only at a temperature of 80 °C.
Before you start processing steel, you must first apply a layer of copper - after this, the product is thoroughly cleaned and dried. To perform metallization using a chemical method, you need to make an acid-base solution.
When chrome plating aluminum, the procedure is performed in special vacuum containers - when exposed to high temperatures, the metal begins to evaporate, after which its particles cover the surface.
The procedure for carrying out the chrome plating procedure
To perform chrome plating of parts at home, it is necessary to carry out a number of preparatory procedures, which will ensure high quality processing of steel or copper.
The procedure consists of several main steps:
- Mechanical cleaning. Using special tools and equipment, it is necessary to carefully remove paint, rust, decorative elements and protective films.
- Product insulation. Before proceeding with chrome plating of the surface, it is worth carefully covering any cracks and holes with metals that are resistant to acids.
- Degreasing. This step is necessary to remove residual oil and grease before metallization. For this purpose, water-based chemicals are used (in some cases, organic solutions are used). Before starting work, you need to prepare enamel dishes - in it, over low heat, the solution is brought to the required temperature, after which the parts to be processed are immersed in the liquid.
- Picking. This stage is relevant only for steel and cast iron - the procedure lasts up to 1.5 minutes (current density varies from 24 to 40 A/dm2). For copper, a similar effect is not used.
Careful adherence to all stages of the technology ensures high quality of the finished coating, thanks to which it will successfully serve for a long period of time.
Removing chromium from a surface
In the initial stages of doing the work yourself, there is always the possibility of making a mistake.
To perform chrome plating at home, you need to master the process of removing low-quality coating from the surface.
Steel parts need to be immersed in hydrochloric acid - the chromium will completely dissolve quite quickly. But it is important to consider that this method is not suitable for every material - for cast iron it is worth using a solution prepared on the basis of alkali (the use of chlorine ions is unacceptable).
Scope of technology application
Manufacturing plants use special equipment, but if desired, the procedure can be done with your own hands. This is the optimal solution for steel, copper and similar materials in the following cases:
- Decorative chrome plating improves the aesthetic characteristics of the product and also performs protective functions.
- Restoration of worn parts is used based on the electrochemical method, but this procedure is effective only if the depth of wear does not exceed 1 mm.
- Improved reflective properties - in this regard, chrome loses only to silver and aluminum, which makes it the best choice for creating mirrors.
- Extending the service life of a copper product and increasing its wear resistance. Porous chromium plating is used for surface treatment of internal combustion engines and similar parts.
The thickness of the coating varies depending on its purpose and the characteristics of the metals being processed. Elements made of aluminum, steel, brass and other materials are subjected to similar processing.
Conclusion + video
You should not forget about following safety rules. It is necessary to work in a well-ventilated area, and also use protective equipment for hands, eyes and exposed skin.
At home, for processing copper, as well as other materials, you can use not only specialized containers as the main reservoir, but also an ordinary plastic bucket or jar (if the size of the parts being processed allows).
Source: http://zonametalla.ru/obrabotka/hromirovanie-v-domashnih-usloviyah.html
Chrome plating at home: 5 stages of the process + video
Most often, car enthusiasts resort to chrome plating. However, they are also interested in decorating the home interior. Decorative chrome plating is a profitable business.
Car enthusiasts apply chrome coating to the metal parts of the vehicle body and thus achieve an original design.
With the help of chemical metallization, other goals are achieved: spraying metal onto the wooden components of designer furniture, creating metal-like designs for plastic parts, making original souvenirs from household items, etc.
Chrome plating affects not only the appearance of the surface. It also improves performance properties. The functions of chrome plating are listed below:
- Protective function . The chromium layer has good resistance to sudden temperature changes and improves the physicochemical properties of the coated surface. This layer protects the surface from oxidation, adding strength to car parts and household items.
- Decorative function . As a result of chrome plating, a beautiful and original appearance of the car (or souvenir item) is obtained. Home interior parts (door handles and ceiling cornices) also look much more aesthetically pleasing after chrome plating.
- Restorative function . Chrome plating allows you to extend the service life of the surface being coated (for example, shafts and bushings if the wear depth is less than 1 millimeter). This also increases the service life.
- Increased wear resistance . After chrome plating, an internal combustion engine becomes more resistant to wear, as do various small parts (stamps, dies, measuring tools).
- Improving reflective properties . Chrome plating of mirror reflectors and other elements will increase the visibility of the vehicle at night, and will allow decorative elements to shine, reflecting the sun's rays and lamp light.
Chrome plating technologies
Chrome plating of parts at home can be done using the technologies described below:
- Galvanic or electrolytic method . With this method, chromium atoms from an electrolyte solution are deposited on the surface of the workpiece when exposed to an electric current. This method is the most popular. Its scope of application is very extensive and includes the creation of products with high reflective properties. Galvanic deposition of chromium helps create a high-quality coating that is highly resistant to mechanical and even chemical damage.
- Chemical or catalytic method . It is based on the interaction of reagents and the reduction of chromium from its own salts. Electric current is not needed with this method. The layer initially has a characteristic gray tint and needs to be polished. Chemical chromium plating, due to the presence of phosphorus reagents, makes it possible to apply a high-quality hard layer to products of complex shapes with cavities. Chemical chrome plating at home implies compliance with safety precautions when working with toxic substances.
- Diffusion method . In this case, chromium is sprayed using a galvanic brush. This method is the most compact and most accessible for do-it-yourself chrome plating. Control of the thickness and quality of the coating is carried out only during the chrome plating itself.
DIY chrome plating
Preparation for chrome plating
When doing chrome plating yourself at home, you may encounter the release of toxic and carcinogenic substances. These substances can be harmful to health, so safety precautions must be observed. Before starting work, complete the steps listed below.
- Ensure reliable ventilation of the room in which chrome plating work will be performed. Even an ordinary garage will work well as a testing ground for work. It is advisable to organize forced ventilation if possible.
- Find personal safety equipment (safety glasses and respirators, as well as rubber gloves and an apron).
- Think in advance about the disposal of waste generated during the process. This waste can be quite toxic.
Remember that chromium electrolyte is capable of releasing volatile compounds that come into good contact with organic matter. In this case, organic matter may be destroyed as a result of such contact.
Such compounds are especially dangerous for the skin, organs of vision and breathing. Goggles and a respirator are the minimum for chrome plating at home.
To carry out chrome plating at home, you will need the following tools:
- Galvanic bath. It is a plastic, polyethylene or glass vessel that is resistant to aggressive environments. To improve the quality of electrolysis, it is necessary to increase the thermal insulation of the bath. To do this, it is enough to place it in a box, upholstered on the inside with material with additional insulation.
- A power source having the following characteristics: the ability to adjust the input voltage, the presence of a wire cross-section at which the cathode and anode are connected to the current source (must be at least 2.5 square millimeters), a current strength of 50 Amperes, a permissible voltage of 12 Volts, and total power no more than 1 kilowatt.
- Heating device for electrolyte. An external heater is suitable for this, which should also be resistant to aggressive environments.
- A thermometer pre-calibrated to one hundred degrees Celsius.
- A lid hermetically attached to a vessel with electrolyte. It should not be metal.
- A lead plate is immersed in a container, and the cathode is attached to a chrome-plated sample. The part is placed in the electrolyte so as not to touch the walls, bottom and anode.
Composition, technology and rules for preparing an electrolytic solution
This mixture contains: distilled or boiled tap and filtered water, chromic anhydride (CrO3) in the amount of 250 g per 1 liter of water and sulfuric acid (H2SO4) in the amount of 2 - 2.5 grams per liter.
Preparation is carried out as follows: the vessel is half filled with water heated to 60 degrees Celsius, then chromic anhydride is poured in, which must be completely dissolved. After this, the remaining water is added and the acid is carefully added. The entire solution is mixed.
The electrolyte must be kept for three and a half hours at rated current.
The quality and durability of chrome coating depend on proper surface preparation. The chrome surface must be cleaned. During cleaning, dirt and any foreign bodies are removed from the surface first. For cleaning, you can use either regular sandpaper or a grinding machine (depending on the degree of contamination).
In addition, the surface must be degreased. A special solution is perfect for this. It includes: sodium hydroxide (150 grams per liter), silicate glue (5 grams per liter) and soda water (50 grams per liter). The solution is heated to a temperature of 90 degrees Celsius. The product is kept in the solution for 20 minutes.
If the surface is covered with a large layer of fat and dirt, the exposure time can be increased to an hour.
Do-it-yourself chemical metallization at home proceeds as follows:
- The electrolyte must be heated to a temperature of 52 degrees Celsius and then maintained at this temperature.
- It is necessary to place a part with an attached cathode into a vessel with a pre-attached anode and heat everything until the temperatures are equalized.
- Voltage must be applied. Settling time can vary from 20 minutes to an hour. It all depends on the shape of the surface.
- It is necessary to remove the part and rinse it in distilled water, and then dry it for 3 hours. During drying, the surface should not come into contact with dirt (including it should not be touched with hands, even with gloves). This is how steel, brass and bronze surfaces are chromed.
It is especially worth highlighting chrome plating of plastic at home. Chrome must be applied to plastic in a well-ventilated area, since such a process is prohibited by residential legislation. As a result of chrome plating, plastic will look more sophisticated and its resistance to damage will increase.
For this type of chrome plating, a galvanic brush is used. The bristles, about 25 millimeters in diameter, should be tightly wrapped with lead wire. It is fixed at the end of a cylindrical vessel filled with electrolyte. A diode is attached to the other end. The electrical circuit uses a step-down transformer.
Its minus is attached to the chrome surface. The plus is directed to the anode of the diode, and the cathode of the diode must be connected to the bristle winding. Next, liquid is applied to the surface to be treated using uniform movements. Each section must be brushed at least 20 times.
At the end, the element is processed and dried, and the dirt is removed using a compressor.
Features of the technology
When performing preparation, it is necessary to take into account that the polishing of the product before chrome plating must be carried out efficiently. Chemical reagents must be measured in precise proportions. When preparing the electrolyte, it is allowed to use chemically pure sulfuric acid.
Chromic anhydride is more difficult to find, so you will have to tinker with it for a long time to find it.
- The shine on the coating is uneven. The reason may be that the current supplied to the anode and surface is too high. Another reason is incorrect electrolyte temperature.
- There is no shine. This is caused by a lack or excess of chromic anhydride. Also, the reason may lie in an insufficient amount of sulfuric acid or in an exceeded operating current rating.
- The appearance of brown spots on the surface. You have definitely exceeded the content of chromic anhydride in the solution. A lack of sulfuric acid may also have an effect.
- The appearance of small shells. The surface was poorly polished, and hydrogen was not removed from it.
- The chrome plating is uneven. This occurs if the operating current has been exceeded.
- The applied coating is too soft. In this case, the current strength, on the contrary, was underestimated, and the electrolyte temperature did not reach the required values.
- Peeling of chrome plating. In this case, there was an unstable voltage, and the surface was not completely degreased. The electrolyte temperature may have decreased during the process.
Results
To avoid the above defects, strictly follow the instructions for chrome plating surfaces at home.
Article rating:
(1
Source: https://motorsguide.ru/tuning/hromirovanie
How to do chrome plating at home
Is it possible to do chrome plating at home? The answer to this question is quite ambiguous, since this process is fraught with many difficulties, which can only be solved with knowledge in the field of chemistry and chromium plating technology.
In order to correctly and safely carry out the chrome plating process with your own hands, you should take into account all the features of the chemical and physical transformations that take place in the galvanic bath.
Most of the reagents involved in chrome plating are substances that are particularly hazardous to health, so before you start experimenting with chrome coatings, carefully study the theoretical side of the process.
Next, we will try to consider in detail the chemical component of the issue, safety measures and how to make a galvanic bath and electrolyte.
Chrome plating is a physical and chemical process during which a thin layer of metallic chromium is deposited on the surface of the workpiece or part.
This metal gives the surface a shiny appearance, making the chrome plated product look very beautiful.
Electroplating opens up wide opportunities for improving the decorative, physical and chemical properties of materials.
Chrome is extremely resistant to aggressive environments; it does not tarnish or darken under the influence of water and air, which is why it is widely used in the design of car body parts and parts of mechanisms operating in difficult conditions.
Decorating car body parts with chrome
The thickness of the chrome coating is very small: from 0.075 to 0.25 mm. Unlike nickel, chromium is not applied directly to the metal in most cases. To do this, use a thin layer of electroplated substrate. This sublayer consists of copper or nickel and requires the use of additional technological operations, complicating the already difficult chrome plating process.
Another difficulty that can stop a home craftsman from completing the task is the purchase of chemical reagents. The main component of chrome plating is chromium oxide (CrO3), another name is chromic anhydride.
An unpleasant feature of its use is that hexavalent chromium oxide is a strong poison, the lethal dose of which for humans is about 6 g. This chemical compound has a limited circulation, strictly controlled by the state.
The waste generated after the completion of chrome plating must be disposed of in accordance with a special procedure, and not simply poured into the sewer, or worse, into the soil.
Chromic anhydride is a carcinogen; when its solution gets on the skin, very strong irritations occur, including eczema and dermatitis, which can develop into skin cancer.
When chromium oxide combines with organic substances (oil, gasoline, etc.), fires and explosions occur. This substance is extremely dangerous to health and life, so before starting work you should weigh the pros and cons, assessing the feasibility of such a decision.
The first thing you need for chrome plating is a well-ventilated area separate from living quarters. You should not start experimenting at home in the kitchen, bathroom or other places not intended for chemical equipment.
The best choice would be a large garage or workshop, which should first be emptied of containers with gasoline, oil, paint and solvents. It would also be a good idea to equip a forced ventilation system.
Be sure to get a fire extinguisher and consider an emergency fire exit option.
Chrome plating equipment includes:
- galvanic bath made of plastic;
- rectifier with parameters 12V/50A;
- acid-resistant heater;
- thermometer.
In addition to the galvanic bath, you will need several additional containers of the same size for washing the workpiece. To save time and money, it will be necessary to organize a separate galvanic bath for copper or nickel plating, since constantly changing reagents in one container is time-consuming and impractical.
Galvanic baths for copper or nickel plating
The straightener must be quite powerful, especially if you want to chrome medium-sized and large-sized parts with your own hands.
Make your calculations based on the fact that to create a shiny surface, a current density of the order of 15-25 A/dm2 is required, so that a conventional rectifier can ensure normal operation of the process, maximum for car door handle linings or small interior trim parts (gearbox knob, radio casing rim) , and so on). Large parts - wheels or a bumper - will most likely not be possible to cover with chrome yourself, or it will cost an amount commensurate with the purchase of new spare parts.
As for the heater, some sources recommend using a regular heating element. I would like to strictly warn you regarding this solution, since chrome plating requires equipment that is resistant to acids; a heating element is not such a device, and its use will, at best, lead to breakdown of the electrolytic bath.
The most common thermometer can be used, with divisions from 0 to 100°C. The temperature at which the process proceeds uniformly is 47-52°C; the main task will be to establish and maintain stable these parameters throughout the entire reaction time.
The chrome plating process is carried out by galvanic means. To carry it out, it is necessary to have a cathode, an anode (workpiece) and an electrolyte, in the environment of which chemical reactions will occur.
Assembling a device for chrome plating is quite simple, especially if you have previously had experience in creating copper or nickel coatings: the technology is similar, only the environmental parameters, electrolyte composition and cathode material differ.
A sheet of lead or its alloy with tin is used as a cathode. It is best for the lead plate to be slightly larger in size than the workpiece. The cathode is connected to the positive electrode of the rectifier.
The anode is connected to the material that should be chrome plated. It must be “suspended” in the electrolyte medium in such a way as not to touch the walls, bottom, and in no case touch the cathode.
Chromium plating of material in an electrolyte environment
Creating an electrolyte requires the presence of the following components:
- chromic anhydride, at the rate of 250 g/l of electrolyte;
- sulfuric acid – 2-2.5 g/l;
- distilled or pure water, without iron impurities.
Before making the electrolyte, heat the water to a temperature of 60-80°C, then dissolve chromic anhydride in it. Cool the mixture slightly and add the required amount of pure sulfuric acid in a thin stream. The acid should not be technical, but pure and concentrated.
Electroplating is very sensitive to the composition of the electrolyte, so at chromium plating enterprises there are entire laboratories that constantly monitor the stability of the reagents. When chrome plating yourself, you will have to do without the help of chemists and technologists, but if there are few parts to be processed, then the composition of the electrolyte should change uncritically.
Self-plating with chromium is impossible without proper preparation of the surface of the product. First you need to create a copper or nickel substrate, since chromium will not lie on the surface of steel, aluminum or any other metal.
Copper plating or nickel plating is carried out in a galvanic bath, in which the cathode is metallic copper or nickel, respectively, and the electrolyte is a solution of sulfuric acid and copper sulfate or nickel salts.
After preparation is completed, the product is carefully ground and polished, taking care not to damage the thin layer of the substrate, degreased and dried.
Self-plating with chrome
Self-chrome plating must take place under stable parameters of voltage, temperature and electrolyte composition. Any deviation may lead to coating defects.
For example, exceeding the current concentration per unit area leads to the formation of growths and dendrites of metallic chromium on the sharp corners of products.
Violation of the temperature regime, as well as fluctuations in the concentration of reagents, cause darkening, dullness or spotting of the coating.
After chrome plating of parts at home is carried out, the chrome surface is covered with a sufficient layer of metal, the voltage is turned off, the product is disconnected and placed in a bath with distilled water. It is better to repeat the process several times, changing the water each time.
Before you start chrome plating yourself, you should carefully evaluate the future cost of the work and draw a conclusion about its feasibility. If you do not have a place to conduct such experiments: for example, your garage or workshop, then you should not start, otherwise you can greatly harm others.
Before making a chrome plating device, think about and plan for future waste disposal.
When released into groundwater and then into wells, chromium oxide causes poisoning and the development of cancer, so it is strongly recommended not to begin work without first deciding on all the intricacies of the process.
Source: http://tutmet.ru/oborudovanie-hromirovanija-domashnih-uslovijah-svoimi-rukami.html
The process of chrome plating metal products at home

Chrome plating can change the quality and decorative composition of any item. Many companies offer metallization services for elements, but there is an alternative to the expensive procedure. It is quite possible to carry out chrome plating at home if you know some of the subtleties and rules of production technology.
Chrome plating procedure
Chrome plating is a complex physical and chemical process that involves mirror silvering of individual elements using sputtering.
The chromium coating is resistant to oxidation and aggressive pressure from the external environment, and also retains the richness of the shade for a long time.
Chrome plating is used not only to update car parts, but also to create interior decor.
Conditions for the procedure
The process of transforming nondescript parts into mirrored objects involves the use of chemical reagents. A living room or kitchen is not suitable for chrome plating at home.
For such an experiment, it is worth creating an impromptu laboratory in a garage or other isolated room.
Dangerous fumes of reagents can increase the risk of cancer, so the selected room must be equipped with ventilation, and the technician must be equipped with protective clothing, goggles and a mask.
Preparation of the workplace is a very important part of the work, during which it is necessary to take into account some features of the technology.
Under what conditions is it safe to carry out chrome plating?
Basic equipment
The main tools used in metal processing include the following components:
- electrochemical bath or glass container;
- current rectifier;
- a heating element;
- thermometer.
Diagram of an electrochemical bath for chrome plating
Using a regular thermometer, you can maintain the temperature required for the procedure. Chemical reagents are the main participants in the process of silvering a metal. The main component is chromium oxide, which in a certain dosage is considered a deadly poison. The use of this substance should be treated with extreme caution.
Chemical reagents
Do-it-yourself chrome plating also involves finding reliable suppliers who sell the necessary substances. Most chemical components can be purchased at medical equipment warehouses, while the rest can be purchased at pharmacies. The starter kit includes the following substances:
- AgNO3 – silver nitrate – 2 g;
- SnCl2 – tin dichloride – 2.5 g;
- Glucose – 2.5 g;
- NaOH – sodium hydroxide – 22 g;
- NH4OH (ammonia) – 5 ml;
- HCl – hydrochloric acid – 20 ml;
- Formalin 37% – 0.45 l;
- Distilled water – 2 l.
Using a kitchen scale or measuring cups, you can measure the dosage of each drug. And for the process of chrome plating itself, you will need disposable syringes and household sprayers.
Coating manufacturing technology
The technology of metal pollination with chromium includes 4 stages.
Preparation of solutions
This stage of work begins with the production of a special composition from chemical components. A solution of tin chloride will be needed to activate the metal surface. It can be prepared using the following ingredients:
- distilled water – 0.5 l;
- tin chloride – 2.5 g;
- hydrochloric acid – 20 ml.
Preparation of solutions for chrome plating
The reducing agent recipe requires the following set of components:
- distilled water – 0.5 l;
- formalin – 5 ml;
- glucose – 2.5 g.
Recipe for silver solution:
- distilled water – 0.5 l;
- silver nitrate – 2 g;
- sodium hydroxide – 2 g;
- ammonia – 5 ml.
Surface preparation
Preparing a product for chrome plating with your own hands requires degreasing the surface of the metal part. Recipe for degreasing composition: distilled water – 0.5 l (to – 50-60o) and sodium hydroxide – 20 g.
The coating should be wiped with a degreasing liquid, then the solution should be thoroughly rinsed off. Unprocessed fragments of the part will not be amenable to diffusion metallization.
Surface degreasing
Coverage activation
The metal pollination reaction must be activated. Performing this technique is required so that the silver covers the desired object with a reliable layer.
The coating is activated using a solution of stannous chloride for exactly 1 minute. Next, you should cool the surface of the metal by placing it in cold water for 3 minutes.
Failure to comply with pollination time intervals leads to defective parts.
Placing metal in solution
Metallization
Obtaining the desired silver film on the surface of an object is the most interesting stage of chrome plating at home. The silver solution and the reducing agent should be sprayed over the object so that the two compositions lie evenly on the metal surface.
Spray metallization
Methods
Knowledge of the interaction of chemical elements and the availability of basic equipment will help you carry out chrome plating yourself without any problems. There are 3 ways to carry out this procedure.
Galvanic
The electrochemical method of silvering a metal involves the presence of a calcining element - a cathode, a chemical reaction medium - an electrolyte, and a workpiece - an anode.
The electrolyte is a mixture of purified water, sulfuric acid and chromic anhydride (CrO3). The metallization object is immersed in the electrolyte at a water temperature of 60-80°C.
As a result of the reaction, chromium cations settle on the walls of the object, and the part is covered with a mirror film.
Electrochemical device diagram
Catalytic
The chemical method of metal processing is based on the interaction of reagents with the surface of the product. Atoms with high potential rise to the top layer of the coating as a result of reaction with reagents. The resulting coating is polished and brought to a mirror finish.
Diffusion
This method of chrome plating retains the basic principles of chromium deposition, but the procedure itself is carried out using a galvanic installation.
This type of do-it-yourself chrome plating eliminates the need to build a bulky electrolytic bath.
The galvanic installation is a special brush into which an electrolyte, a transformer and a cord connecting the cathode and anode are poured. Instead of the usual fluffy brush, you can use a porous sponge.
Reasons for the appearance of defects on the surface of parts
Experienced craftsmen identify several main reasons that affect the quality of chrome plating at home.
Methods for eliminating defects on the surface of parts
Excess current . Excessive current passed through an object may result in uneven coloring of the object.
Failure to comply with technology . If the optimal temperature parameters and dosage of reagents are violated, the metal will not acquire the desired mirror shine.
Poor preparation of the base product . Poor degreasing will not allow chromium cations to settle evenly on the surface of the object, and will also reduce its service life.
Related video: Chemical metallization (chrome plating)
Source: https://VtorExpo.ru/galvanika/protsess-hromirovaniya-v-domashnih-usloviyah.html